
Client Success Stories and Solutions for our Customers
Welcome to Junmu’ Client Success Stories section, where we proudly showcase our completed orders and solutions spanning medical and laboratory equipment. Here, you’ll gain valuable insights into our diverse product range, satisfied customers, and the regions we serve. We believe transparency builds trust—and these case studies reflect our commitment to excellence in both medical and laboratory fields.
cSSD
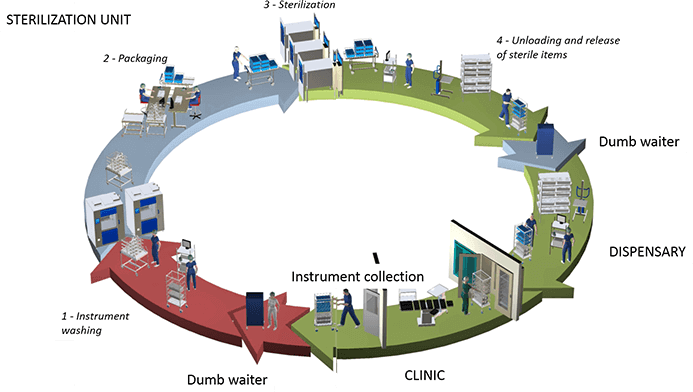
The Disinfection Supply Center Management Traceability System is a series of software products developed by Junmu Medical based on years of experience in hospital infection control and combined with digital information technology, which not only meets the urgent need for improving CSSD management in Chinese hospitals but is also tailor-made for China’s Ministry of Health’s latest standards. It focuses on providing a comprehensive management solution for sterile supply in hospitals and is committed to promoting the development of hospital infection prevention and control, thereby minimizing the risk of infections.


